Why does glass break so easily?
The short answer
Glass breaks easily because its atoms form a rigid, disordered network rather than an ordered crystal lattice. When under stress, there's no way for planes of atoms to slip past each other, so its molecular bonds break easily and form cracks.
The long answer
Glass is ubiquitous in our everyday life. In fact, you're staring at it right now on your phone or computer screen.
Wow, what a great looking website!
Yet for as long as humans have incorporated glass into our lives, scientists are still puzzling over the details of this solid, yet fragile, substance.
Let's start with the basics:
What is glass?
When you cool a liquid, it hardens. Water becomes ice. Lava becomes rock. Molten metal becomes solid metal. Glass also hardens when it cools, but it looks very different at an atomic level.
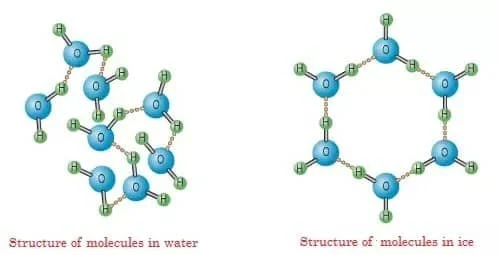
Most liquids harden into a solid through crystallization, in which their molecules go from a free-flowing, disordered state to an organized, repeating pattern.
Compare the disorderly water molecules (left) to the orderly ice molecules (right).
Source: Our Winter World
How cool is this 3D simulation of water molecules turning into ice crystals!
Source: CSIRO’s Data61
Glass is different. When you cool glass, its disorganized molecules start contracting, crowding a bit together, and eventually stop moving. But they never form the repeating pattern structure that make up crystalized solids. In essence, solid glass looks like a "frozen" liquid at an atomic level.
Quartz and glass are both made up of silicon and oxygen molecules, but their arrangement of atoms is different. Quartz (left) crystallizes when it hardens, forming a repeating structure. Glass (right) retains a liquid-like disorderly molecular structure even when it hardens.
Glass, however, is not a liquid. If it were a liquid, you could squish it into new arrangements to take the shape of a container, but its molecules are truly rigid. Glass is a special type of solid material: On the outside, it acts like a solid, but on the inside it appears like a liquid.
Why does glass break so easily?
Glass is considered to be a brittle substance. That means it tends to break, without deforming, when under stress. A metal spoon will bend before it snaps, but a glass straw will shatter without any bend. Why is that?
The secret to glass' brittleness lies in its amorphous (i.e. non-crystalline) molecular structure. Unlike most solids which have molecules organized in neat stacks, the silicon and oxygen atoms that make up glass are connected at random angles in an irregular pattern.
While some of the molecules are closely clustered, the clusters themselves don't have as much force holding them together. That means glass is riddled with weak points.
And since glass, unlike a crystalline solid, doesn't have planes of atoms that can slip past each other when met with force, there is no way to relieve stress. Instead of bending, the stress causes the clusters of molecules to separate and form a crack. As the crack grows, more bonds break and the glass fractures even more.
This is why a broken piece of glass is so sharp. Its edges can be as thin as a single molecule. Ouch.
How do you make glass stronger?
Tempered glass undergoes extreme heat and high-pressure cooling to make the outer layers of the glass cool and solidify faster than the inner layers. The surface layer compresses while the interior stays in tension, giving tempered glass its unique strength.
Read more about how tempered glass works in this previous article I've written.
Curious about how the world works?
Today You Should Know is a free, weekly email newsletter designed to help you learn something new every Friday.
Subscribe today 👇
Check out some other curious questions:
Sources
Fiveable. (2024, July 31). Brittleness – College Physics I – Introduction. https://fiveable.me/key-terms/intro-college-physics/brittleness
Glazing Refurbishment Ltd. (2024, March 26). Why Does Glass Break? (2024 Guide on Causes & Prevention). Glazing Refurbishment. https://www.glazingrefurbishments.co.uk/blog/why-does-glass-break
Puiu, T. (2014, April 25). Glass molecules jam to form fractal wells. ZME Science. https://www.zmescience.com/science/chemistry/glass-molecules-energy-fractal-wells-432543/
Purdue University. (n.d.). Glass and Other Ceramics. Purdue University Department of Chemistry. https://chemed.chem.purdue.edu/genchem/topicreview/bp/materials/ceramic4.html
Reimers, R. (2024, February 15). How We Make Glass Nearly Unbreakable … With Science. YouTube. https://www.youtube.com/watch?v=jd35LNtsX-c&ab_channel=SciShow
Rohrig, B. (2015, April 1). Smartphones: Smart Chemistry. American Chemical Society. https://www.acs.org/education/chemmatters/past-issues/archive-2014-2015/smartphones.html
van Genugten - de Laat, H. (n.d.). The glass phase: a physics mystery. Eindhoven University of Technology. https://www.tue.nl/en/news/features/the-glass-phase-a-physics-mystery
WellnessOptions. (n.d.). Glass: a mysterious material. WellnessOptions. https://wellnessoptions.ca/glass-mysterious-material.html
Wolchover, N. (2020, March 11). Ideal Glass Would Explain Why Glass Exists at All. Quanta Magazine. https://www.quantamagazine.org/ideal-glass-would-explain-why-glass-exists-at-all-20200311/







We Americans sure do like American things.